
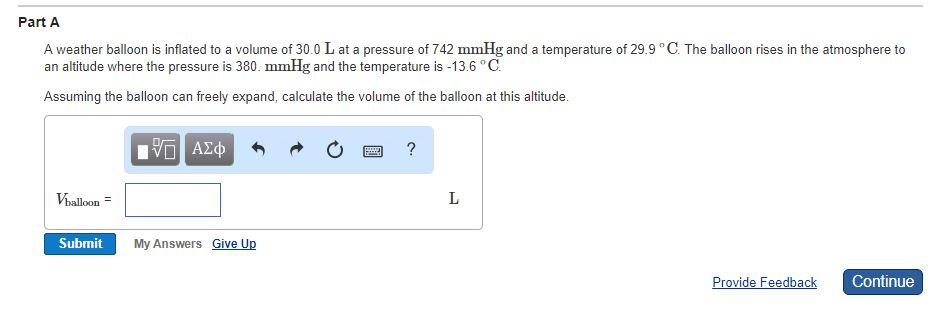
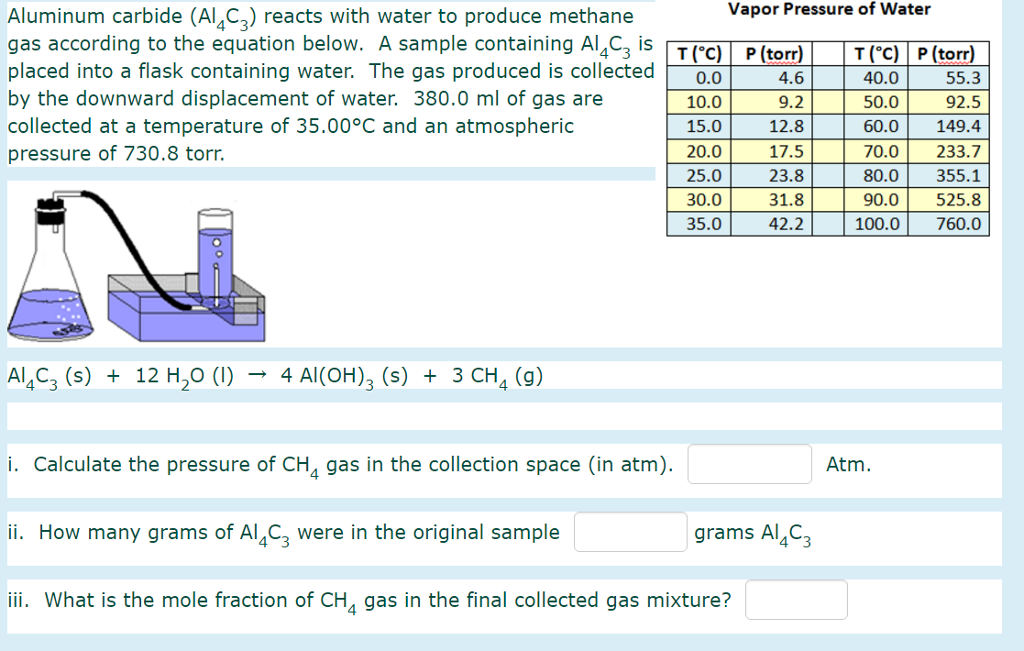
(1 atm 760 mm Hg 101.3 kPa 1.013 bar) asked in Chemistry by xyzagain. Convert the pressure 2.50 atm to kPa (1 atm 101.3 kPa) a.
This is simple to use online converter of weights. Three samples of gas each exert 740 mmHg of pressure in separate containers. Antoine Equation: 10 1175.817log (100 C)6.88555- 3.26601 100+224.867 1845 mm Hg Hexp p p y P pHex Hex Hex 0.150(2.00) atm 760 mm Hg. The pressure value 380 mmHg (mm of mercury) in words is three hundred and eighty mmHg (mm of mercury).
380 MMHG TO ATM MAC
MAC is analogous to the pharmacologic effective dose (ED 50) of drugs. Note that rounding errors may occur, so always check the results. MAC is inversely related to anesthetic potency and, therefore, with its lipid solubility (Meyer-Overton theory). MAC has been determined in different age groups, under different conditions, and for all inhalation anesthetic agents ( Table 62-1), allowing for comparison of the potency of the different agents. This conversion is done by dividing 30.1 mmHg by 760.
380 MMHG TO ATM SKIN
Minimum alveolar concentration (MAC) is the alveolar concentration of an inhalation anesthetic agent at 1 atm and at steady-state concentration necessary to suppress a gross purposeful movement in 50% of patients in response to a skin incision. When 30.1 mmHg is converted to atm, there are 0.0396 atm in it. The pressure p in atmospheres (atm) is equal to the pressure p in. Therefore, a method for quantifying the amount of inhalation agent necessary for anesthesia has been devised. What would be the new pressure if a 400 mL gas sample at 380 mm Hg is expanded to. 1 millimeter mercury (mmHg) is equal to 0.00132 atmospheres (atm). However, for inhalation anesthetic agents, the mass of drug and patient weight have little to do with the intensity of the drug effect. Please visit pressure conversion to convert all pressure units.CHAPTER 62 Minimum alveolar concentrationĭosing for most drugs is based on mass of drug per kilogram of patient body weight. What is the final pressure of the gas,in atm. To convert torr to atm, multiply the torr value by 0.00131578947 or divide by 760.Ītm (atmospheric pressure) is the force per unit area by the weight of air above that point. Solution for The volume of a gas with an initial pressure of 380. Ideal gas constant, R 0.082057 L atm mol K. If 22.5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature. To convert atm to torr, multiply the atm value by 760.
380 MMHG TO ATM HOW TO
How to convert atm to torr (mmHg)?ġ Atm (atmospheric pressure) is equal to 760 torr (mmHg).

Alternatively, to find out the torr value for the most commonly converted atm value, you may check the atm to torr conversion table.īelow, you will find information of how to convert atm to torr and how to convert torr to atm, including the formulas and example conversions. To convert torr to atm, multiply the torr value by 0.00131578947 or divide by 760. If 22.5 L of nitrogen at 748 mm Hg are compressed to 725 mm Hg at constant temperature. To convert atm (atmosphere) to torr (mmHg) and to convert torr (mmHg) to atm, you may use the converter above.


 0 kommentar(er)
0 kommentar(er)
